Biolistic delivery with shock-free gene guns
For a number of years now, our group has been designing and building customized gene guns for biolistic delivery of nucleic acids and fluorescent dyes to cells and tissues. The technique of biolistic delivery has been introduced in 1980ies. It uses micron-size carrier particles made of a heavy metal (tungsten or gold) that are coated with plasmids, RNA, or dye, accelerated to a high speed using a pneumatic gun (Gene Gun) and launched into a biological target (cultured cells, tissue, plant, small animal…). A small bead penetrates cells without damaging them, gets stuck in a cell in an internal layer of the targeted tissue, and releases into the cells the chemicals that it carries. The technique of biolistic delivery has been used for transfection (beads coated with plasmids), RNAi (dsRNA coating), and staining (lipophilic dye coating). Biolistic delivery is fast, contact free, and can, in principle, be performed with minimal damage. In addition, deep tissue layers can be reached independently of tissue properties other than its mechanical rigidity. Gold carrier particles are commercially available in a variety of sizes (0.5 – 1.5 um) and with surface coatings optimized for DNA, RNA, or lipophilic dyes. Two gene guns are commercially available from BioRad. The most popular model, Helios™ is a handheld shotgun with a price of ~$17,000. Biolistic delivery has been successfully used to transfect plants, some tissues, and cultured cells.
The current range of applications of the technique of biolistic delivery is limited because of multiple problems with the performance of the available gene guns.
1. Gas jet damage. Whereas the perforation of the targeted tissue by ~1um gold beads may be tolerable, the jet of high-speed compressed Helium emerging from the gun barrel is highly damaging. Various solutions have been used to slow down the jet, mostly at a cost of reduced penetration depth, but none is completely satisfactory for targets other than plant leaves.
2. Poor aiming and limited consistency and reproducibility. A hand-held gun is a poor match for small targets. The targeted region is ~1 cm in diameter. Most particles stop near the top surface of the targeted tissue and their penetration depths are widely spread. The particle loading is not well controlled and their number varies from shot to shot.
3. Limited penetration depth and the use with rodent brain. The targeting of neurons in soft mammalian brain tissue is a natural application for biolistic delivery. Impressive staining of neurons in brain slices has been achieved. In addition, transfection and penetration depths up to 200um into extracted brain tissue were demonstrated with a modified BioRad gun, but the tissue was somewhat damaged. The present use of biolistic delivery in neuroscience is very limited and no useful biolistic delivery into live mammalian brain has ever been demonstrated.
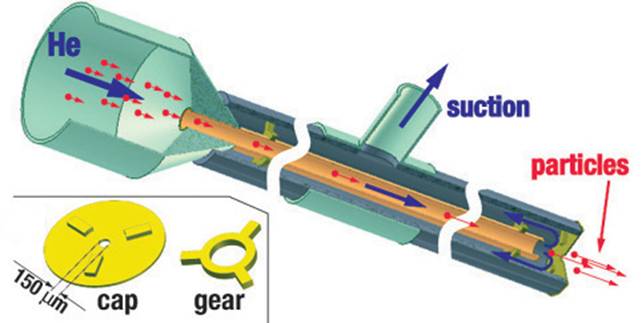 In 2004 we built and tested a new
type of gene gun with the barrel made of a narrow capillary (ID = 0.5 mm)
and enclosed by a coaxial tube of a larger diameter (OD = 2.5 mm). In this
capillary gene gun, the flow of Helium emerging from the inner capillary
(barrel) is entirely diverted into the outer capillary, with no gas flowing
from the gun nozzle (150 um orifice) at any time. The diversion of Helium that
flows at ~1 km/s is achieved by the application of vacuum suction to the outer
tube and is verified by placing a dish with water right under the nozzle (with
no ripples observed on the water surface).
In 2004 we built and tested a new
type of gene gun with the barrel made of a narrow capillary (ID = 0.5 mm)
and enclosed by a coaxial tube of a larger diameter (OD = 2.5 mm). In this
capillary gene gun, the flow of Helium emerging from the inner capillary
(barrel) is entirely diverted into the outer capillary, with no gas flowing
from the gun nozzle (150 um orifice) at any time. The diversion of Helium that
flows at ~1 km/s is achieved by the application of vacuum suction to the outer
tube and is verified by placing a dish with water right under the nozzle (with
no ripples observed on the water surface).
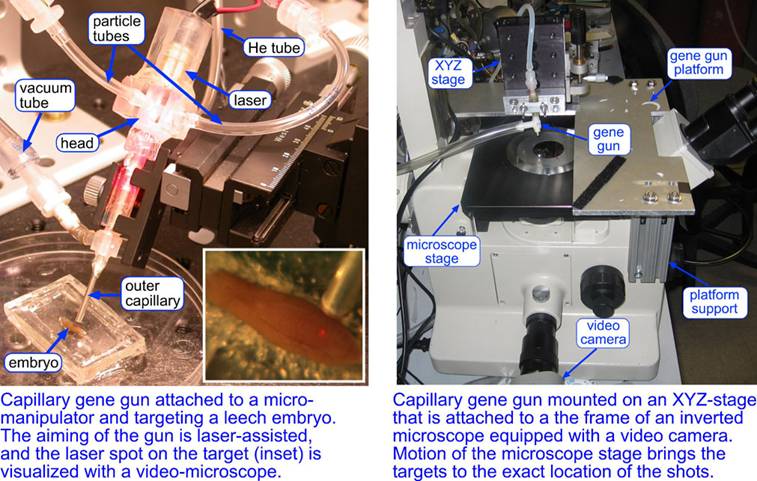
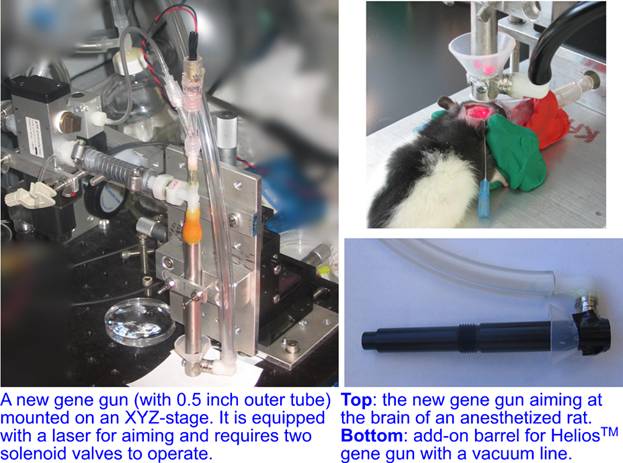
The capillary gene gun resolved two major issues of the biolistic delivery: the damage to the target from the gas shock wave and the aiming precision. An aiming precision of ~100um can be readily achieved by mounting the gun on a micromanipulator, aiming the shots with a laser beam shining through the barrel, and observing the target under a low-resolution video microscope, which is suitable for thick non-transparent targets (e.g., leech embryos). With this setup, we have been able to perform RNA-interference, transfection, and fluorescent staining of neurons and muscle cells in internal layers of leech embryos. The aiming precision has been improved by mounting the gun on an XYZ-stage attached to the frame of an inverted microscope equipped with a video camera. The location of a test shot into a blank target (agarose gel) is market on the video monitor, and the biological targets are brought to the marked position using mechanical stage of the microscope. This setup provides an aiming precision of ~10um and can be applied to thin transparent targets (cultured cells, tissues, brain slices, C. elegans worms…)
Nevertheless, the particle penetration depth achievable with the capillary gene gun has remained rather limited, <100um into a 3% agarose gel (used to mimic a soft tissue) for 1.6 um gold beads, which was comparable with the performance of the Helios™ gun at less than optimal settings, substantially smaller that with Helios™ gun equipped with a modified barrel, and apparently insufficient for targeting neurons in live rodent brain. Therefore, we have recently overhauled the capillary gene gun, substantially increasing its size and changing its mode of operation. The new gene gun retained the original feature of zero gas shock wave, while achieving ~2.5× greater penetration depth into agarose gel, ~270um with 1um gold beads and ~80um with 0.5um gold beads. In addition to a stand-alone version of the new gene gun, which is mounted on an XYZ-stage and can be used with an inverted microscope, we have also built an add-on barrel for the Helios™ gun that suppressed the gas shock wave (up to a pressure of at least 75 psi).
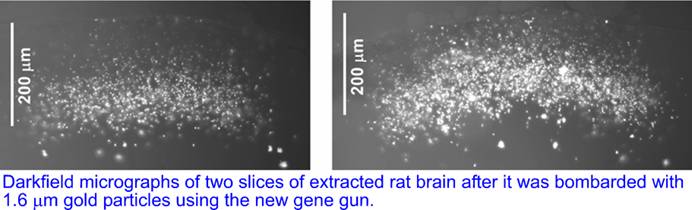
We tested the new gene gun on extracted rat brain with 1.6 um uncoated gold particles. The brain was vertically sliced post-assays and the slices revealed the delivery of gold particles to depths up to 200um with most of the particles found at depths >100um, corresponding to cortical layers 1-2.
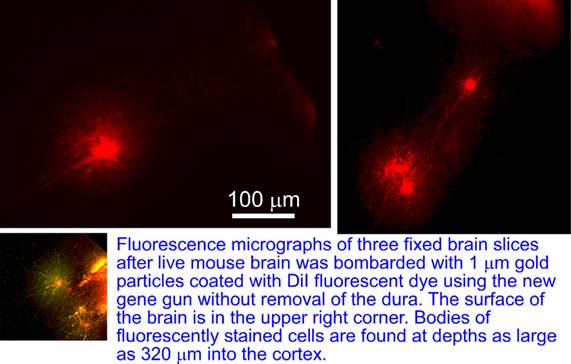
The gun was further used to deliver 1 um size gold beads coated with DiI lipophilic dye into the brain of anesthetized mouse without the removal of the dura matter covering the brain. After the brain was sliced and imaged, fluorescently stained neurons were observed at depths of up to 320um into the brain.
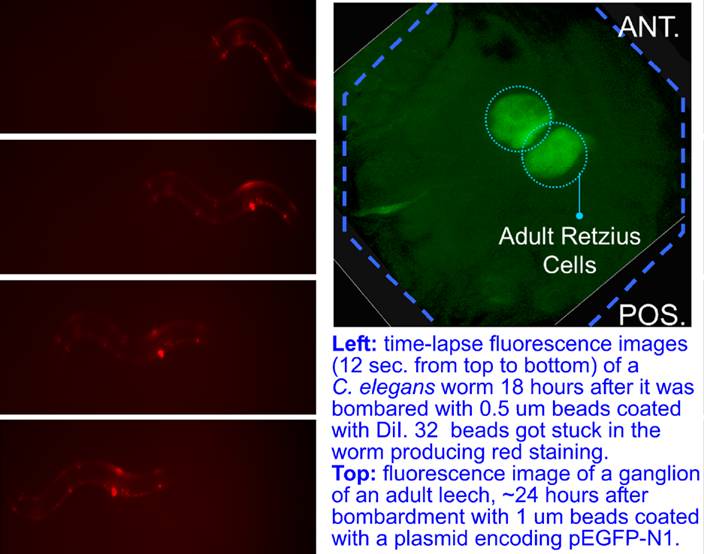
Finally, the new gene gun has been used to launch 0.5um beads into C. elegans worms. The bombarded worms with an average number of 5 beads in them had an excellent 48 hours post-assay viability. In one case, a worm with 32 beads inside remained apparently healthy and moved at a normal speed. The gun was also used cells in ganglia of an adult leech (Hirudo medicinalis). A small and shallow window was opened above the ganglia on the ventral side by cutting away just enough stocking to reveal a faint outline of the ganglia under the dissection microscope. The beads shot into the ganglia had to penetrate the thin layer of stocking, the sinus, and the capsule to reach viable neurons, to enable transfection without significant exposure of the ganglia to external environment. 1 um beads were coated with a plasmid encoding pEGFP-N1. 24 hrs after the bombardment, strong green fluoresence was observed in homologous Retzius cells in leech ganglia #12.